The third quarter was a pivotal period for both the biotech and pharmaceutical sectors, with regulatory developments and an increase in business deals shaping the landscape for the industries.
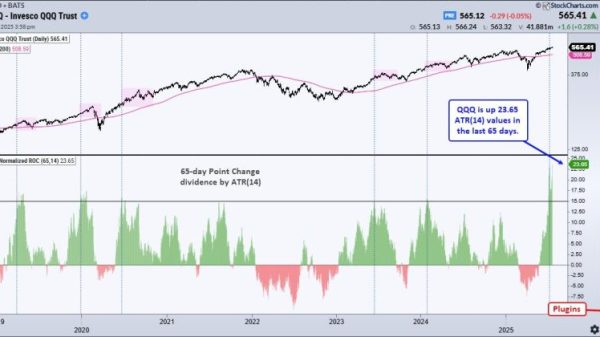
Public biotech indexes rallied above critical levels last seen in 2021, with the NASDAQ Biotech Index (INDEXNASDAQ:NBI) closing 21 points ahead for the quarter and up 11 percent year-to-date.
Emerging artificial intelligence (AI) applications are becoming increasingly critical in drug discovery and R&D, highlighted by products like AlphaFold and new draft guidance from the US Food and Drug Administration (FDA) that encourages AI use in regulatory submissions. However, cautious funding approaches remain, especially for early stage companies.
This confluence may signal a sector resurgence, despite continued funding caution for early stage firms.
Biopharma M&A activity picks up
In a Q3 report on M&A activity, Oppenheimer notes that biopharma market sentiment showed an upward trajectory during the quarter, with expectations that deal flow will continue to increase through the end of 2025.
William Blair, a global investment banking and asset management firm specializing in biopharma investments, also notes an uptick in momentum in a recap of Q2 activity in the biopharma space, citing positive clinical data, a wave of public M&A activity and more clarity on tariffs and drug pricing as catalysts.
Total M&A transaction value reached US$38 billion for the quarter, according to data analyzed by Oppenheimer, including US$20 billion in September alone. Clinical-stage acquisitions saw their strongest quarter since late 2023, driven by early stage assets in the oncology, immunology and cardiovascular-metabolic areas.
The central nervous system space saw a pause in deals for the first time since the beginning of 2024, reflecting shifting investment priorities. Small molecules and antibodies maintained their leading positions as prevalent treatment modalities in deals, while emergents like bispecific antibodies, multi-specific antibody-drug conjugates and CAR-T therapies gained traction. However, the overall M&A market for antibody-drug conjugates remained cautious, with the exception of Seribant Therapeutics’ acquisition of Y-mAbs Therapeutics for US$412 million.
Public company takeouts continued to outnumber private company acquisitions for the second consecutive quarter; however, private companies still attracted strong interest from investors after a sluggish first half of 2025.
Oppenheimer’s Private Placement Activity report notes that a significant increase was observed in September, with companies with a clinical pipeline and a platform commanding the highest valuations.
Strategic partnerships between established pharmaceutical leaders and innovative biotech firms continued to underscore the ongoing efforts by pharma leaders to build and diversify their pipelines.
Roche Holding (OTCQX:RHHBY,SWX:ROG) and Zealand Pharma (OTC Pink:ZLDPY,CPH:ZEAL) entered into an agreement to co-develop and co-commercialize weight-loss drug candidate petrelintide in a deal valued at up to US$5.3 billion, reflecting ongoing interest in weight-management therapies, despite market challenges and competitive pressure.
Meanwhile, Bristol-Myers Squibb (NYSE:BMY) and BioNTech (NASDAQ:BNTX) agreed to co-develop and co-commercialize a novel cancer immunotherapy targeting multiple tumor types in a deal worth up to US$11 billion, and Pfizer (NYSE:PFE) partnered with 3SBio (OTC Pink:TRSBF,HKEX:1530) to advance a new cancer drug candidate.
Both agreements highlight ongoing efforts to expand oncology treatment options.
Cell and gene therapies continued to draw investor attention, and the central nervous system space saw an increase in average deal size. William Blair notes that cell and gene therapies remain a priority area for venture capital investors, as well as public market investors, despite regulatory complexities.
Initial public offering (IPO) activity rebounded meaningfully in Q3 after a quieter first half of 2025, with LB Pharmaceuticals’ (NASDAQ:LBRX) September offering serving as a marker of renewed capital markets appetite.
Secondary public offerings and clinical-stage private financings also increased, fueled by promising clinical data and expanding investor participation, including from international markets such as China.
In parallel, funding for AI-driven drug discovery platforms continued to capture investor interest, with rounds for companies like Isomorphic Labs, Pathos and Lila Sciences.
Regulatory and policy developments
US President Donald Trump’s second term has brought a shift to more business-friendly stances, impacting healthcare M&A and trade. The Federal Trade Commission has signaled intentions to ease antitrust scrutiny, potentially speeding up big pharma and biotech dealmaking and encouraging higher transaction volumes that consolidate the sector.
A central policy focus is the onshoring of biopharmaceutical manufacturing, with the administration actively pursuing tariff negotiations to reduce import costs and bolster supply chain resilience. The landmark deal between the government and Pfizer to lower drug prices in Medicaid in exchange for tariff relief exemplifies this dual approach.
These tariff adjustments are designed to ease the burden on drug importation costs, incentivizing companies to invest more domestically while managing global supply chain risks. Lara Castleton, US head of portfolio construction and strategy at Janus Henderson Investors, has identified this agreement as “the catalyst for healthcare.” She further suggests that the sector is likely overdue for a comeback, having lagged behind the tech market earlier in the year.
Trump has emphasized the expectation that other pharma companies will follow suit, intensifying onshoring efforts. As of September 30, large pharma had committed roughly US$368 billion to US-based manufacturing facilities.
Additionally, the FDA approved 45 new drug applications in Q3, marking a notable increase from previous quarters. This surge was driven by accelerated approvals, largely in the gene and cell therapy sectors, as well as innovative biologics targeting rare diseases and oncology.
Biotech and pharma market forecast for 2025
The biotech and pharma sectors entered Q4 on firm footing. Supportive market dynamics are expected to persist as the year continues, with 2025 on track to reach US$93 billion in total transaction value.
Several catalysts are poised to shape the healthcare landscape moving forward.
An anticipated IPO from MapLight Therapeutics, focusing on neurology therapies, will reveal investor appetite for specialty pharma assets in a market that had a bullish close to Q3, but faces questions about sustaining momentum.
On the regulatory front, FDA decisions are expected for a handful of treatments in gene and cell therapy, as well as oncology. Approvals are expected to accelerate, bolstered by programs aimed at speeding up evaluations of novel treatments like CRISPR-based medicines, stem cell research and nutraceuticals.
Leadership changes may also foster innovation in unconventional medical fields such as stem cell research and nutraceuticals. Amid an evolving regulatory and political landscape, Reed Jobs has advocated for sustained public funding to fuel biomedical progress, delivering a key congressional address on National Institutes of Health protection in September. Beyond advocacy, he is also building a nearly US$1 billion biotech fund focused on next-generation cancer therapies, highlighting the vital intersection of public research funding and private sector innovation.
Policy clarity around drug pricing reforms and Medicaid tariff relief will critically influence commercial access and pricing dynamics. The GLP-1 sector remains under the spotlight following the announcement of Trump’s plans to reduce the monthly cost of GLP-1 drugs like Ozempic and Wegovy to US$150.
AI’s expanding role in drug discovery, clinical trial design and digital therapeutics will continue to inspire industry innovation, likely attracting significant funding and fostering new collaborations.
However, volatility related to regulatory appointments, trade uncertainties and notably the ongoing US federal government shutdown presents near-term challenges. Investors and industry participants will closely monitor clinical data and regulatory shifts to navigate the evolving landscape successfully.
Securities Disclosure: I, Meagen Seatter, hold no direct investment interest in any company mentioned in this article.